Please select your Region.
Please select your Region.
Mar 01, 2019
To prevent the loss of blood through injuries and other causes, organisms possess two abilities: the ability to form blood clots*1 to stanch the flow of blood (blood coagulation), and the ability to break those clots down (fibrinolysis). In a healthy organism, both of these abilities are performed in a balanced manner.
Blood loss is a major cause for serious complications during the perioperative (preoperative, intraoperative, postoperative) period, and blood loss is related to more than half of the deaths which occur within 30 days of the perioperative period. For this reason, blood coagulation tests are performed as a part of homeostasis management.
However, the items in a conventional coagulation test (prothrombin time*2, fibrinogen*3, etc.) are tested using plasma samples after centrifugation in a central laboratory. Thus, there was a need to wait for the results.
There is also the issue that coagulation factors other than plasma components cannot be determined, meaning that the in vivo state is not accurately reflected.
ARKRAY's new "SPOTCHEM HS HS-7710" is an instrument intended to comprehensively grasp the state of a patient's blood coagulability. Fully-automated measurement can be started via a simple operation of setting a whole blood sample, reagent cartridge and pipette. In heart surgery, liver transplantation and child birth, there is a high risk of severe blood loss. Fast, accurate hemostasis management is needed in surgical sites and intensive care units, and it is environments like these where the "SPOTCHEM HS HS-7710" can be of service by allowing doctors to grasp the changes in their patient's coagulative condition.
Previously, ARKRAY has developed blood and urine analysis systems mainly for diabetes testing and expanded its business in the clinical testing industry. The "SPOTCHEM HS HS-7710" is the first comprehensive blood clotting analyzer successfully developed by a Japanese manufacturer. With the development of this instrument, ARKRAY has succeeded in breaking into the surgical field and plans to further expand their business in the future.

This device was developed by ARKRAY with the support of The Japan Agency for Medical Research and Development (AMED) and applies technologies*4 developed by Sony Corporation.
○Usage of whole blood
Unlike standard blood coagulation tests which use centrifuged plasma, by using whole blood samples it is possible to perform coagulability evaluations approximate to the in vivo state (the blood itself). This makes it possible to determine the factors causing the bleeding. Additionally, no pre-processing of the sample is necessary, leading to shortened processing times.
○Convenient measurement
Using five types of cartridges, a maximum of four items can be measured at once. Also, the instrument is equipped with automated sample dispensing, reagent stirring, and temperature control features, meaning that fully automated measurement can be started by merely setting a whole blood sample, reagent cartridge and pipette.
○ Coagulation time, strength and fibrinolysis time measurement
Blood coagulation time (the ease with which clotting occurs), blood clot strength (the ease with which bleeding occurs), and fibrinolysis time (the time it takes for clots to dissolve) are measured. These can be used as accurate judgment indicators for the administration of blood transfusions.
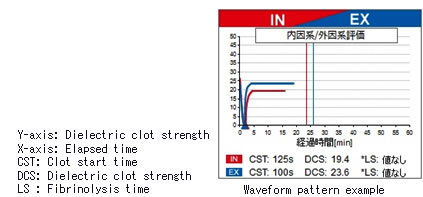
○Visual determination of condition changes
Changes in coagulability over time are displayed on the instrument's panel as a wave form. Using comparisons between items and past data, etc.,
changes in the patient's condition can be visually apprehended.
By combining five types of purpose-specific reagent cartridges, coagulation start time, maximum coagulation strength and fibrinolysis time can be measured.
| Test Item | Function | |
|---|---|---|
| IN (Intrinsic coagulation test) | Intrinsic coagulation factors are stimulated, and coagulation is elicited | |
| HN (Extrinsic coagulation test) | Intrinsic coagulation factors are stimulated after heparin has been neutralized, and coagulation is elicited | |
| EX (Extrinsic coagulation test) | Extrinsic coagulation factors are stimulated, and coagulation is elicited | |
| LI (Fibrinolysis inhibition test) | Extrinsic coagulation factors are stimulated under fibrinolysis inhibition, and coagulation is elicited. | |
| CA (Recalcification coagulation test) | Chelated calcium is recalcified, and coagulation is restarted | |
During the coagulation-fibrinolysis process, if an electrode is introduced to blood and the dielectric constant is measured, that constant will increase with coagulation. Utilizing this mechanism, an alternating current is introduced to a whole blood sample undergoing coagulation (or fibrinolysis) by means of the constituent in the reagent cartridge, and the dielectric constant of the whole blood sample during the coagulation (fibrinolysis) reaction is monitored. From the rising (or descending) curve of the dielectric constant, coagulation time or the maximum strength of clots are calculated.
*1 A term referring to coagulated blood within a blood vessel.
*2 A test to measure the activity level of blood clotting factors referred to as extrinsic pathways. If the presence of these factors decreases in the blood, the time it takes for coagulation to occur lengthens.
*3 The blood concentration measurement of a blood coagulation factor called fibrinogen.
*4 The dielectric spectrum method was developed under AMED's Development of Advanced Measurement and Analysis Systems Program
| Name | Blood coagulation analyzer "SPOTCHEM HS HS-7710" | |
|---|---|---|
| Release date | March 1, 2019 (Friday) | |
| Specifications | ||
| Measurement target | Citrated whole human blood (Hct: 20~60%) | |
| Measurement item | Comprehensive whole blood coagulation | |
| Measurement principle | Dielectric spectrum method | |
| Measurement time | Up to 75 minutes (depending on measurement conditions) | |
| Measurement sample number | 1 sample (sample tube) | |
| Maximum reagent number | 4 reagent cartridges maximum | |
| Required sample size | 1 mL (1 assay) - 2 mL (4 assays), 3 mL maximum | |
| Meas. Environment | Temperature:10 - 30°C, Humidity: 20 - 80% RH (no condensation)) | |
| External dimensions | 380 (width) × 500 (depth) × 460 (height) mm | |
| Weight | Approximately 27 kg | |
| Power supply | AC100V±10%, 50/60 Hz | |
| Power consumption | 150 VA | |
| Sale price | Preferred delivery price: 6,800,000 yen (before tax) | |
| Registration number | 25B1X00001000057 | |
| Category | Class I (general medical devices)/Specific Maintenance Medical Device | |
The information presented in this release is limited to Japan as of the time of its announcement.
![]()