Please select your Region.
Please select your Region.
Oct 26, 2016
Directed towards the overseas market with diversifying needs, ARKRAY, Inc. (hereafter 'ARKRAY') has newly added GLUCOCARD W to its line-up and shall commence sales from late October 2016. It is a measurement device that meets the requirements prescribed in EN ISO 15197:2015*1 regarding the accuracy of devices and on the effect of interfering substances such as hematocrit*2.
The world's diabetic population continues to rise and as of 2015 it stands at 415 million. It is projected to rise to 642 million by 2040*3. Diabetes may cause complications such as diabetic retinopathy and diabetic nephropathy, so in the interest of prevention, it is important for patients to have proper awareness of their condition and monitor their blood glucose.
In 1970, ARKRAY was the first in the world to succeed in the development of a simplified blood glucose test meter. Through the supply of the GLUCOCARD product line as well as diabetes management systems, ARKRAY has been committed for over 40 years to continually improve QOL for patients and to support professionals at the frontlines of diabetes care who give instructions to patients. ARKRAY will continue to promptly supply products that match the needs in various countries and regions in the world to support the frontlines of diabetes treatment.
•High Accuracy Measurement
Realizes highly accurate test results that maintain accuracy in accordance with EN ISO15197:2015. Corrects hematocrit effect before providing test results.
•Rapid and Simple Measurements
Measuring time is just 7 seconds. Speedy measurement is possible.
•Testing with Micro Sample of Whole Blood
A micro sample (0.5μL) from the fingertip is all that is needed for testing.
•Simple Data Management Using USB
Export data to your PC via USB for simple blood glucose management.
•Equipped with Test Strip Disposal Lever
Used test strips can be discharged by pushing the test strip disposal lever, which allows hygienic and safe use without contacting blood.
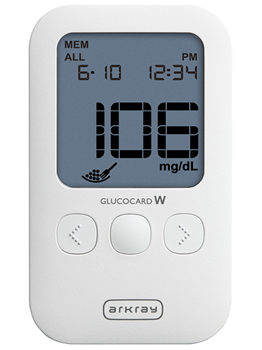
GLUCOCARD W
*1 EN ISO 15197:2015
ISO 15197 is the "In vitro diagnostic test systems -- Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus," a specification for self-monitoring devices for blood glucose that has been used since 2003 as the standard for evaluating device performance
To further improve the accuracy of self-monitoring devices for blood glucose, clinicians were requesting more stringent requirements. In response, the revised edition, ISO 15197:2013 came into effect in May 2013, providing more stringent variance allowances and newly added requirements on the impact of hematocrit, which was not included in the 2003 edition. Citing this revised international standard, a standard was established in Europe as EN ISO 15197:2015.
*2 Hematocrit
The ratio of red blood cells within the blood.
*3 Estimated number of diabetic patients
Source: "IDF Diabetes Atlas Seventh Edition" (November 2015 / International Diabetes Federation)
| Name | GLUCOCARD W |
|---|---|
| Release date | Late October 2016 |
| Specification | |
| Meas. target | Blood glucose level |
| Meas. range | 10 to 600mg/dL,0.6 to 33.3mmol/L |
| Required sample | 0.5 µL (whole blood) |
| Test strip | GLUCOCARD W Test Strips |
| Meas. time | 7 seconds after applying blood |
| Memory capacity | 500 test results |
| Operating environment | Temperature: 8 to 40℃ , Humidity: 20 to 80% |
| Power source | 3V: Lithium flat battery x 1 |
| External dimensions | L84.0×W50.0×17.6 mm |
| Weight | 47g |
| Distribution Territory | Worldwide (excluding Japan) *This product will not be distributed in Japan. |
This product will be sold by ARKRAY Global Business, Inc.
ARKRAY Global Business, Inc. manages overseas distribution for ARKRAY products.
![]()